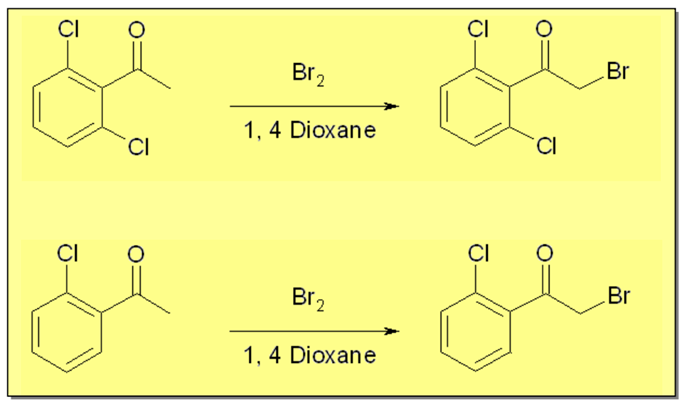
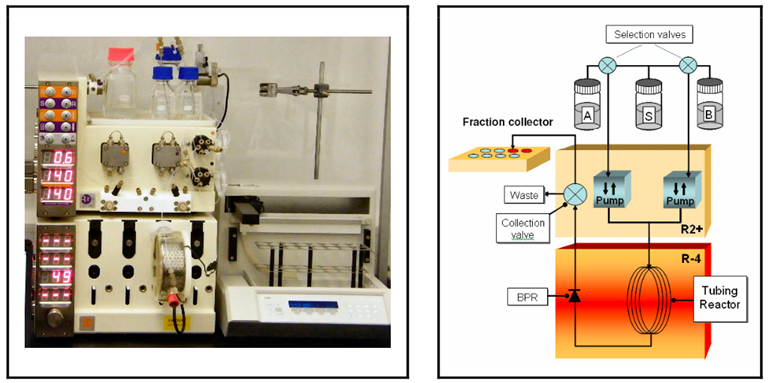
Bromination of Ketones under continuous flow conditions
α-Bromination of ketones is an important reaction in organic synthesis as the resulting α-Bromo- ketones are widely used in organic synthesis as valuable reaction intermediates used in the synthesis of variety of biologically active compounds. Various bromination procedures for carbonyl compounds have been developed, in which the most commonly used protocol involved the use of molecular bromine.
The use of bromine reagent however has some downsides :
– its toxicity and high vapor pressure make it difficult handle
– in most cases selectivity is an issue as undesirable dibrominated side product forms in addition to the formation of HBr as a by-product.
Other alternative brominating agents, such as N-bromosuccinimide and tetralkylammonium tribromides have been used instead of molecular bromine. However, molecular bromine is required in the preparation of these brominating reagents. The general method of α-bromination generally requires slow and careful addition of the bromine reagent into a diluted ketone mixture while maintaining the reaction mixture at low temperature.
Continuous flow chemistry provides excellent controlled addition and mixing of the reaction substrates, in addition to the enhanced heat transfer as a result of increased surface to volume ratio.
We have carried out two examples of α-bromination of acetophenones using the Vapourtec continuous flow system. This system, with the aid of the Flow Commander PC based control software allowed a fast screening of various reaction parameters such as temperature and molar ratio to efficiently find an optimal condition in which high selectivity and a fast complete conversion compared to conventional method were obtained.
Setup:
| 產品型錄
Vapourtec 產品型錄下載
【Vapourtec】官網