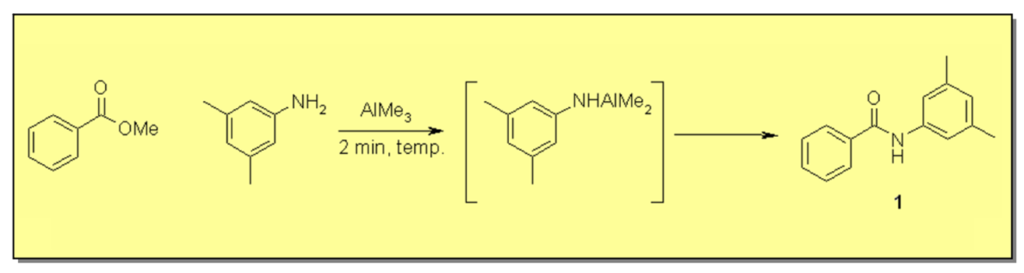
Trimethylaluminium mediated (Weinreb) amide bond formation ¹
This application note illustrates how a reaction which would be subject to thermal runaways and possible explosions in batch mode can be carried out safely using a continuous flow approach.
Weinreb amidation, utilizing trialkylaluminium to activate amines for condensation with esters, is a widely used method for the formation of amide bonds.² This method circumvents the detour of ester hydrolysis, activation of the carboxylic acid and treatment with amine that is otherwise usually employed in amide formation. However, the Weinreb protocol carries some inherent risks that limits its use, especially on larger scale. Trimethylaluminium and tri-isobutylaluminium, the reagent most often employed, are highly pyrophoric and difficult to handle. In addition, the intermediate aluminium-amide that is formed usually carries high energy, making thermal runaways and explosions a risk when applying this protocol. To overcome these problems and hopefully shorten the reaction times (thereby increasing the efficiency of the procedure), we wanted to explore the use of a continuous flow modification of the process. In this study, a benzoic acid derived ester and an aliphatic ester are condensed with an aniline and benzyl amine, respectively. As an application to the above method, we also performed the final step in the synthesis of the anti-obesity drug Rimonabant.³
| 產品型錄
Vapourtec 產品型錄下載
【Vapourtec】官網